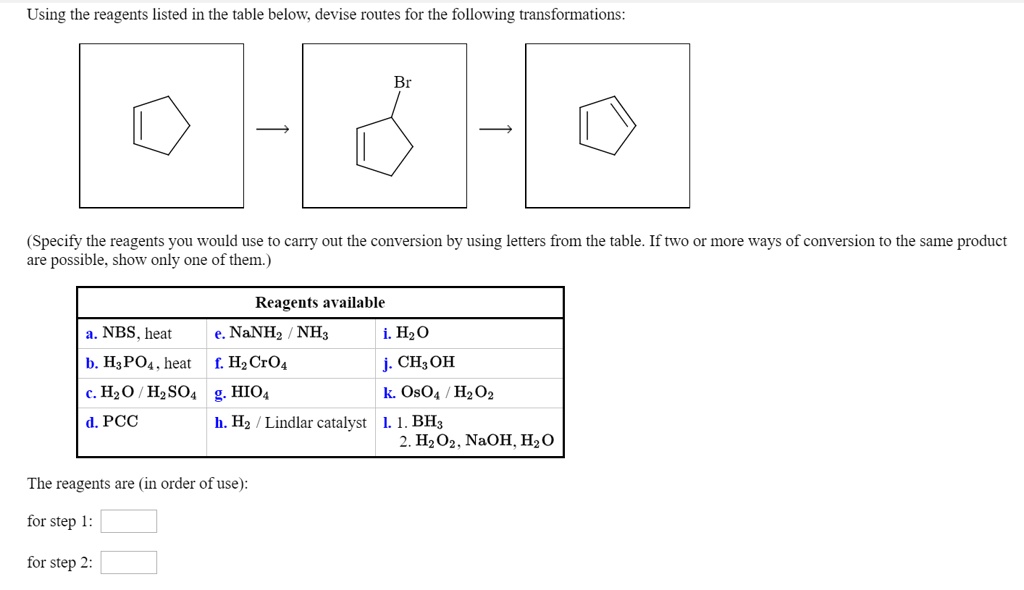
Using the Reagents Listed in the Table Below, Show How to Bring About the Following Conversion:
Question
Using the reagents listed in the table below; devise routes for the following transformations:(Specify the reagents YOu would use to carry out the conversion by using letters from the table: If tWO Or more ways of conversion to the same product are possible. show only one of themReagents available a. NBS , heat NaNHz NH: i. H20 HsPOa _ heat [ Hz CrO4 j: CH; OH Hzo HzSO4 g. HIOs k OsO4 Hz 02 d,PCC Hz Lindlar catalyst L L. BH; 2. Hz O2. NaOH, Hz0The reagents are (in order of use):for stepfor step
Using the reagents listed in the table below; devise routes for the following transformations: (Specify the reagents YOu would use to carry out the conversion by using letters from the table: If tWO Or more ways of conversion to the same product are possible. show only one of them Reagents available a. NBS , heat NaNHz NH: i. H20 HsPOa _ heat [ Hz CrO4 j: CH; OH Hzo HzSO4 g. HIOs k OsO4 Hz 02 d,PCC Hz Lindlar catalyst L L. BH; 2. Hz O2. NaOH, Hz0 The reagents are (in order of use): for step for step 2
Answers
How would you carry out the following transformations? Tell the reagents you would use in each case.
We are forming an Elkin bombing and Elkin and Elkins. So we must perform and elimination elimination reaction. This problem requires requires elimination with elimination with the elimination with specific specific radio chemistry, radio chemistry, chemistry we must form the less Ah substituted substituted Elkin That is the off man product. Therefore, um, therefore, the we will need need thio Thio, um may do use, uh, hysterically, hysterically, terrifically hindered face that is city field dogs side the obss tickle is that and Aurich Group is a terrible leaving, um living group. So we must First, we must first convert the o h group toe better leaving mm group. Mm. We can dio that by converting by converting it into a and, uh, fossil it docile it which will then undergo then undergo elimination. Onda go elimination when traded. All right, Dark. We're dogs. It
Okay you want to create the following products from benzene. And the first one to create is um three Nitrobenzene symphonic acid. So so far nick acid is an S. 03 H. I. Group and it's gonna have priority over and that's your group. So they're both meta directors. We can really add either one in any order. We're gonna add S to three first. So it's A three is going deeper donates sulfuric acid and as a result it becomes better electrified. In the college database of sulfuric acid is gonna deported the benzene room to form a bond back with the addition of the essay through The S. or three s. group. And for nutrition it's just each end of three with sulfuric acid as catalyst to. That's why I want to create is structure. So the how genes are Worth fair directors. We can add either 1 1st super termination, B. R. Two and F. E. Bea arthur is catalyst. So one of the burmese gonna attack the iron and form this complex with br the one burning becoming more ultra silk, the one burning, it's not bound to the iron. And this occurs bees. Irons also trinity of the broom in and um if you BR. Three or somebody's gotta deeper any benzine to restore the arab necessity current chlorination, LCL theories the catalyst and it's a similar mechanism to the art so.
This question is a question asked us to give some theses or each of the following reactions. So the first one is one beauty in going to 13 of you dying. So one beauty mean looks like this. And 13 view design looks like this. Um And so the easiest way to put something on this molecule that we can then eliminate is to use, and B s and peroxide slowed the radical reaction. So if we take our one boutin and we add and B s and rock sides, then we would get I'm actually to products that either get this product or we can get this product because we can move that a little radical around two products here on, then in order to let it get to the dying here, we're going to eliminate. And now this one is really obvious how it gets years of this for me will just eliminate this direction. But we could also do a 14 elimination on this one to get to that same products. So one for elimination is really just the opposite of a 14 addition. So instead of moving the carbon condom, we're adding we'll just move it when we're eliminating. So, um, to get both of these Teoh the product will do that one for elimination. And so to do that and a regular 12 elimination for the other one will use a bulky base like turkey talk side on and then the corresponding alcohol and some heat on. And that will give us 13 beautifying from both of those products in the middle there. Let's part A in part B, we have one pen teen going to 13 di are 13 Penta dying. Um and so here we're gonna do a similar reaction. So we'll start off by adding NBS and peroxide to get to products. So the first one will look like this, and the second one will look like this were removed that and with roaming over here and then we'll do that same thing. So we'll do a 12 elimination here and a one for elimination on this one to get us to the, um, product that we want so well again, use that be talk side with the corresponding alcohol and heat and that will give us the 13 point dying from both of those products for part C here. We're starting with an alcohol, and we need to form two double bonds. So, um, the best way Teoh do that. I think I drew that product wrong. I did. Product that were actually trying to get to is this. There's only one, um, anyone double gone in there. So that looks That's a product that we're trying to get. Sorry about that. Okay, so we're started with an alcohol and going to a double bond on and we need, so we need to eliminate that. Alcohol is our first step. So the best way to eliminate a primary alcohol is with a shoe s a four, and heat on due to rearrangement will actually get the alcohol in the middle, or the double bond in the middle of that chain will get both the cyst entrance products of that. So there's two products here on then to get Teoh. Um, having the bro means on here. This will be a 14 addition from a dying. So we need to turn this into a dying. I will do that the same way that we did in parts A and B. So first, we will add And yes, and rock sides just like we did in Andy. And then we'll eliminate it with the same three agents on. And so then we will get this, um, from both of those. So then we can to get to this product appear, all we have to do is add br two and heat. And the reason that we're adding the heat is to get the thermodynamic product. So this will do a 12 and a 14 reaction. But we only want the 14 products. And luckily, the 14 product in this case is a thermodynamic products. So we can push thermodynamic products by adding heat to the reaction. So what is part C and then for part D. Um, here we have groups for part D. Here we have this internal al Keen going to this only done years out of roaming on. And so there are. There's what one really easy way to do this, and that's just adding NBS and peroxide. But we should also note that that will also give us a second product where we moved the double bond. Um and so liberal mean would be here. So that will give us two products on then for part E here. We're going from just an Al Cane. Teoh. This has a double bond and a bro mean on it. So the first thing that will do is add br two and light because it's the only thing that we can do to an al cane. So that will give us this, uh, cycle fencing with one drumming on it. And then we will eliminate the bro. Mean, it's the same way that we eliminated in the, uh, earlier parts of this problem. So we'll eliminate that, bro mean to get a keen there and then Teoh add the bro Mean will use NBS and peroxide just like we had been doing before. And now, because this, because of this molecule, has the symmetry in it, it will move the double bond on, give two different residents forms, but we'll end up with the same product, the president's form school, actually the same thing as well. Um, because there's nothing else on the molecule to make the many different, so that will only give us one product, but that will be ever seen a product. And then for part F here. All we have to do is eliminate the bro mean to get that second double bond here. So we'll do that the same way that we've been doing it in the last few steps. So used her beauty oxide again with its course when the alcohol is a solvent and he to push the elimination.
Similar Solved Questions
5 answers
Recitation 13: Alkynes and synthesisProvide the products or reagents for the following reactions:Brz / CH,Cl; eicessH-Br leicesHeSO, H,OH; Fdon€HfO,Hz Lludlat" Clle/ Iiq AH,MlacBook Air
Recitation 13: Alkynes and synthesis Provide the products or reagents for the following reactions: Brz / CH,Cl; eicess H-Br leices HeSO, H,O H; Fdon€ HfO, Hz Lludlat" Clle / Iiq AH, MlacBook Air...
5 answers
By using the information in this problem and data given below calculate AH; lor 2,3 AIrimethyipentane_Standard Enthalpies of Formation, JHZ at 298 K Sloltance Formul (kmol) Acctykne CH,G) 26.7 Ammoni NH;(e) 46.19 Bnzcne CH() 49,0 Ckium cutonute Gcom) 1207 Ckium axkk Col) 615,5 Cutbon diaxlk cok) 393,5 cola) 110.5 Cubon monoxkk Dlamond cW) 8168 Ethane Chm) Ethanol CH,OH() 52.J0 Ehyknc CHG) CH,0.m) 127} Gluos HBr(g) 962} Hydrgca bromidSubatanctFormu [2(Himo) 9290 26160Hydrogen chlor& Hdrger Il
By using the information in this problem and data given below calculate AH; lor 2,3 AIrimethyipentane_ Standard Enthalpies of Formation, JHZ at 298 K Sloltance Formul (kmol) Acctykne CH,G) 26.7 Ammoni NH;(e) 46.19 Bnzcne CH() 49,0 Ckium cutonute Gcom) 1207 Ckium axkk Col) 615,5 Cutbon diaxlk cok) 39...
Using the Reagents Listed in the Table Below, Show How to Bring About the Following Conversion:
Source: https://itprospt.com/num/3108912/using-the-reagents-listed-in-the-table-below-devise-routes